
By continuing to use this site, you agree to our use of cookies as described in our Cookie Policy.
FYLNETRA® is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is a substance produced by the body. It stimulates the growth of neutrophils, a type of white blood cell important in the body’s fight against infection.4
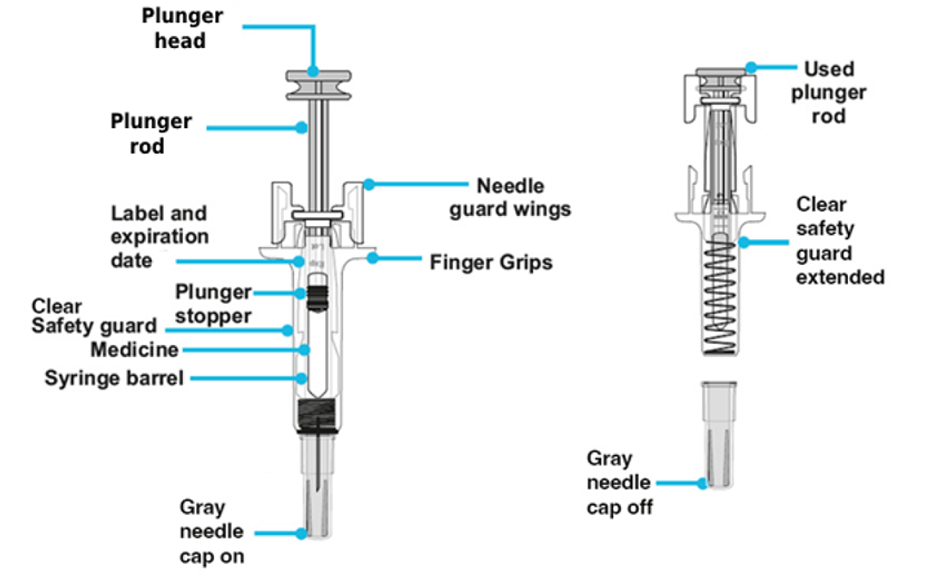
Important: The needle is covered by the gray needle cap before use.
Important: Read the Patient Information for important information you need to know about FYLNETRA® before using these Instructions for Use.
A. Remove the prefilled syringe pack from the refrigerator. Put the original pack with any unused prefilled syringes back in the refrigerator. Remove the syringe tray from the pack. On a clean, well-lit surface, place the syringe tray at room temperature for 30 minutes before you give an injection.
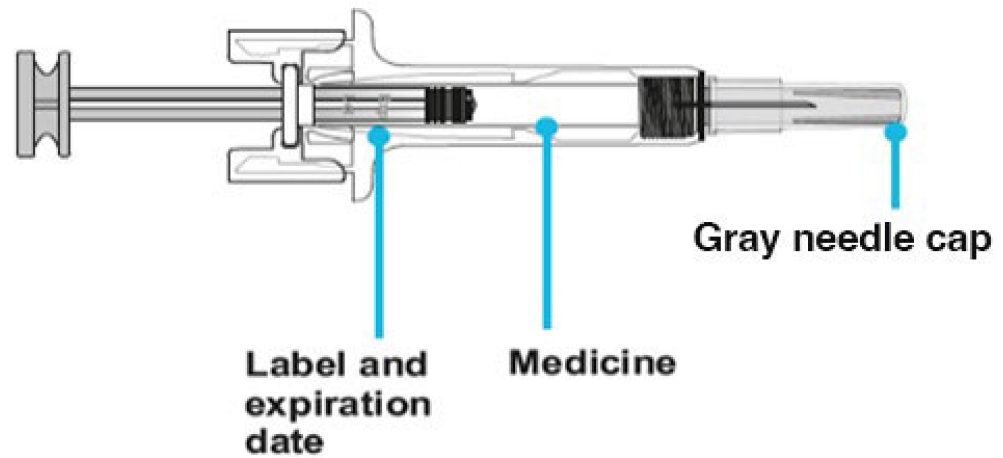
B. Inspect the medicine and prefilled syringe.
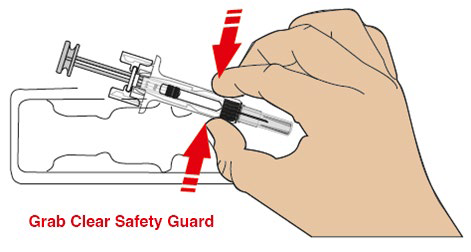
For safety reasons:
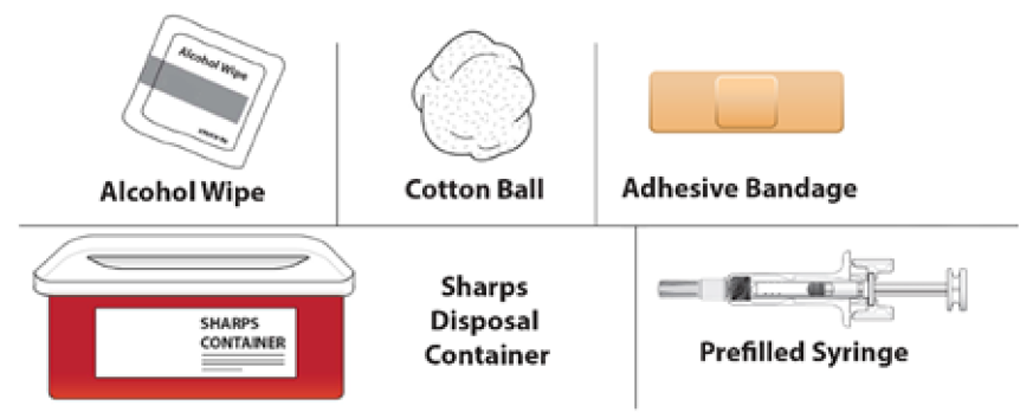
C. Gather all materials needed for the injection. Wash your hands thoroughly with soap and water. On a clean, well-lit work surface place the:
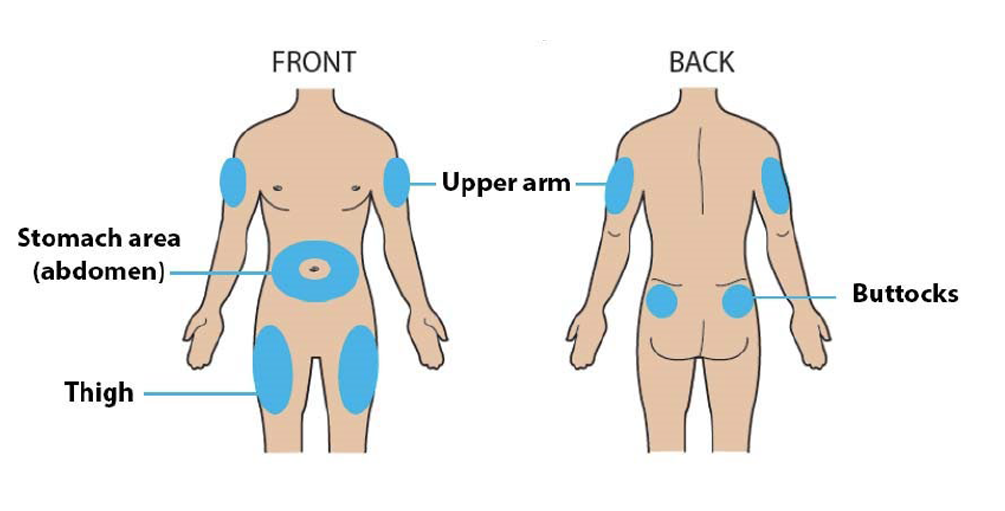
D. Prepare and clean the injection site(s).
E. Hold the prefilled syringe by the barrel. Carefully pull the gray needle cap straight off and away from the body.
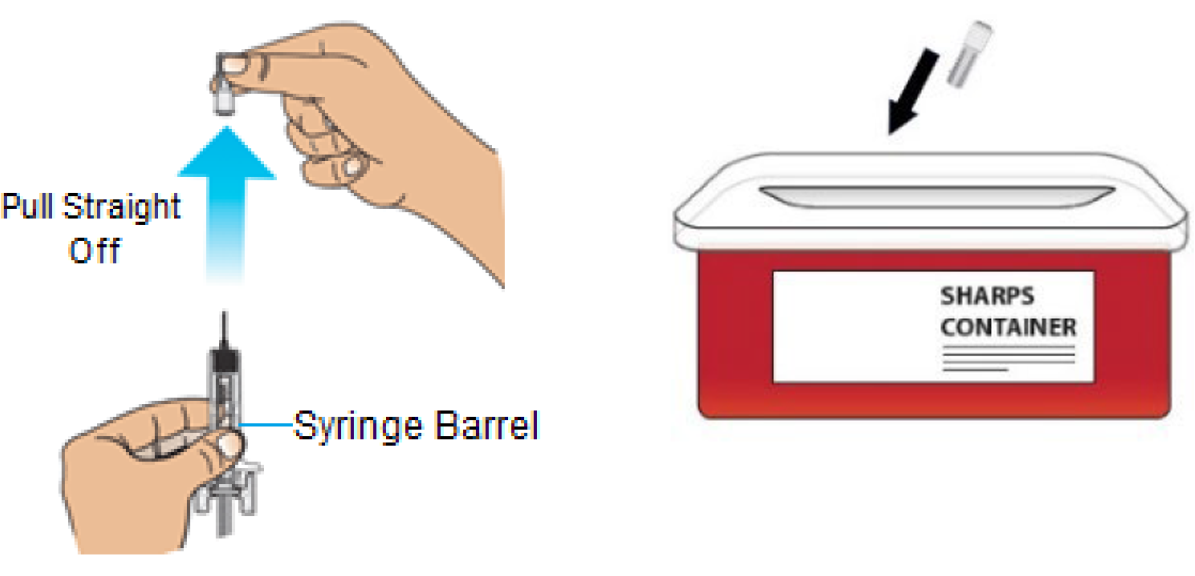
Important: Throw the gray needle cap into the sharps disposal container.
F. Pinch the injection site to create a firm surface.
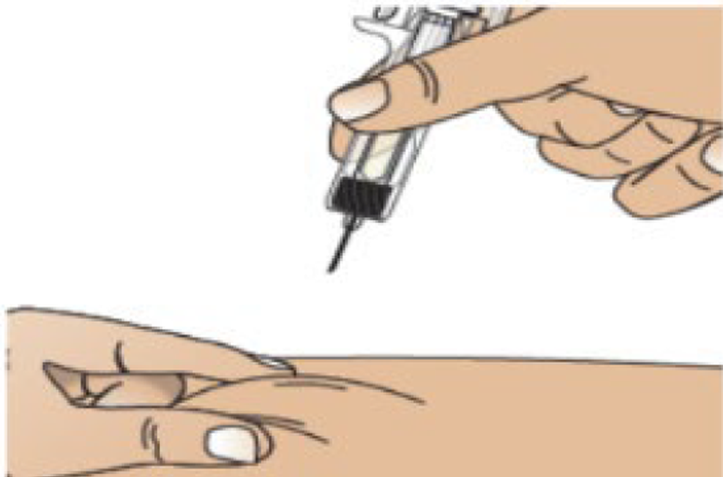
G. Hold the pinch. Insert the needle into the skin at 45 to 90 degrees.
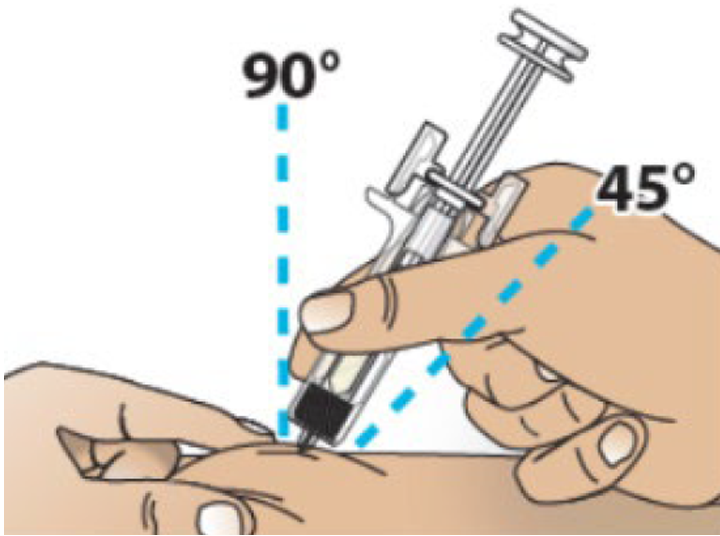
H. Using slow and constant pressure, push the plunger rod until it reaches the bottom and the plunger head is completely between the needle guard wings.
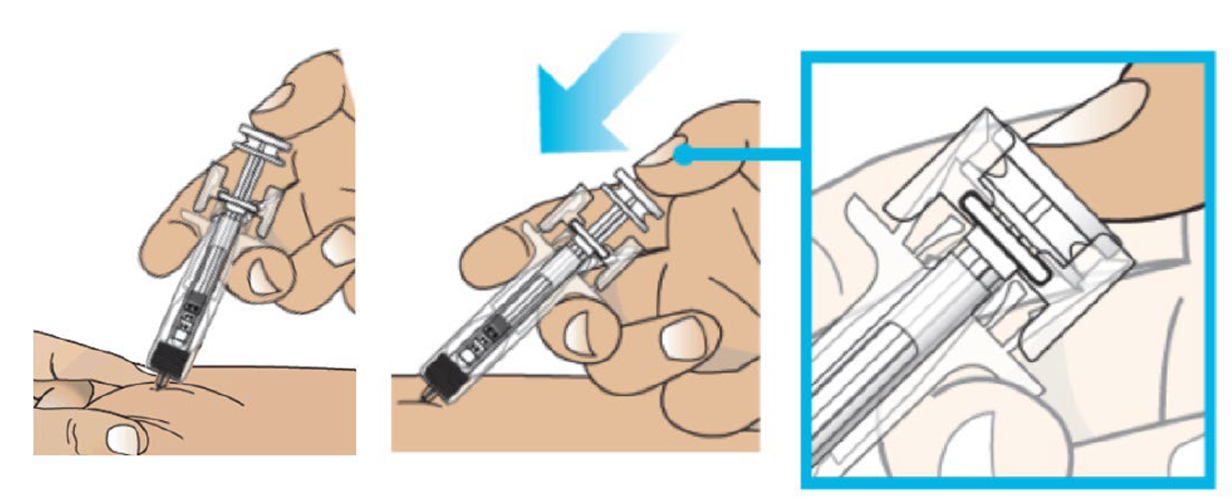
Important: When you remove the syringe, if it looks like the medicine is still in the syringe barrel, this means you have not received a full dose. Call your healthcare provider right away.
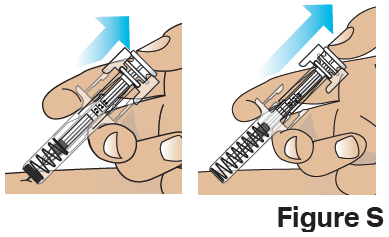
I.

Before you finish!
J. Discard (throw away) the used prefilled syringe.
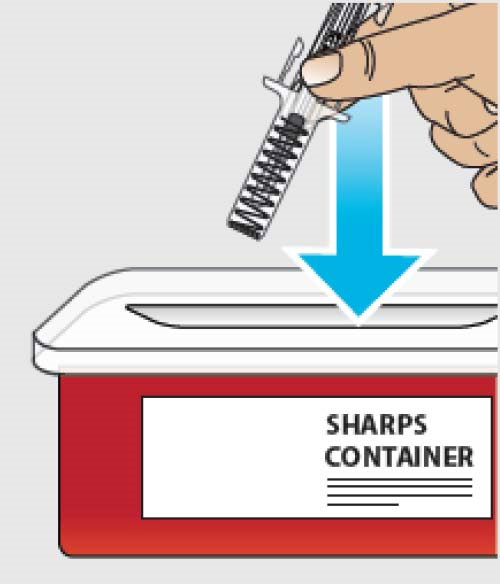
Important: If the clear safety guard does not activate after Step I., remove the needle from the skin and throw away (discard of) the used prefilled syringe as instructed in Step J. right away.
K. Examine the injection site.
If there is blood, press a cotton ball or gauze pad on the injection site. Do not rub the injection site. Apply an adhesive bandage if needed.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Kashiv BioSciences, LLC
Piscataway, NJ 08854
US License No. 2131
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 05-2022-00
PATHways® provides you with a straight-forward range of resources and services designed to easily map your personal path toward more accessible medication. Learn what is available to you and how to keep things moving along your individual treatment path.

It is important that patients do all they can to protect themselves against infection during chemotherapy. Read more about why this is and what you can to help prevent unnecessary exposure.
An infection is when germs such as bacteria, viruses, or fungi get into your body and multiply, causing you to feel ill or damage your organs. The immune system within your body is normally able to prevent and fight infections. Chemotherapy impairs your immune system by reducing the number of infection-fighting cells called neutrophils that are able to protect you from getting sick.
Sepsis is a life-threatening complication where the body is unable to fight infection. Patients with cancer who get an infection are at risk for developing sepsis.
What are the signs and symptoms of sepsis?4
Use this handy acronym to of the word sepsis itself to help remember the signs.
S – Shivering, fever, or very cold
E – Extreme pain or general discomfort (“worst ever”)
P – Pale or discolored skin
S – Sleepy, difficult to rouse, confused
I – “I feel like I might die”
S – Short of breath
The following precautions are suggested as preventative measures:
References:
1. Fylnetra® Summary Basis of Approval – Drug Approval Package https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761084Orig1s000TOC.cfm
2. Food and Drug Administration, FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Guidance for Industry, 2015.
3. Food and Drug Administration, FDA. Draft Guidance: Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations, 2019.
4. Fylnetra® Full Prescribing Information – https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eeadd641-573d-47fe-897a-61006e5f9e03