
By continuing to use this site, you agree to our use of cookies as described in our Cookie Policy.
FYLNETRA® is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.
FYLNETRA® is contraindicated in patients with a history of serious allergic reactions to pegfilgrastim products or filgrastim products.
Multiple in vitro analytical similarity studies using state-of-the-art orthogonal analytical methods demonstrated similarity between FYLNETRA® and Neulasta® in key features such as: primary structure, molecular conformation, charge variants, protein content, purity, and biological activity.1,4
Comparative stability studies between FYLNETRA® and Neulasta® under accelerated and forced degradation conditions have demonstrated similar behavior and degradation pathways for the two products.1,4
FYLNETRA® clinical comparability included data from 120 healthy subjects evaluated in 1 PK/PD study and 230 healthy subjects in 1 immunogenicity/safety study:
1. Strong comparability PK package in healthy volunteer clinical study: The PK similarity of both products was established for serum filgrastim AUC0-t; AUC0-inf and Cmax.1,2
2. Strong Comparability PD package in healthy volunteer clinical study: As a surrogate for clinical efficacy, PD similarity of both products was established for baseline-corrected absolute neutrophil count (ANC) AUEC0-t and Emax for ANC:1,4
References:
1. Fylnetra® Summary Basis of Approval – Drug Approval Package https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761084Orig1s000TOC.cfm
2. Food and Drug Administration, FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Guidance for Industry, 2015.
3. Food and Drug Administration, FDA. Draft Guidance: Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations, 2019.
4. Fylnetra® Full Prescribing Information – https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eeadd641-573d-47fe-897a-61006e5f9e03
FYLNETRA® is a biosimilar filgrastim which has been developed in comparison with Neulasta® as the Reference Product (RP).
Multiple state-of-the-art analytical methods were applied to evaluate all quality attributes required of the biosimilar and confirmed analytical similarity between FYLNETRA® and Neulasta®1:
The same amino acid composition is confirmed in FYLNETRA® and Neulasta®.
In the figure below, the same set of twelve (12) primary peptides G1 to G12 are seen from the digestion of FYLNETRA® or Neulasta® by Glu-C for peptide mapping.
Representative Peptide Mapping Chromatograms* of FYLNETRA® and Neulasta®
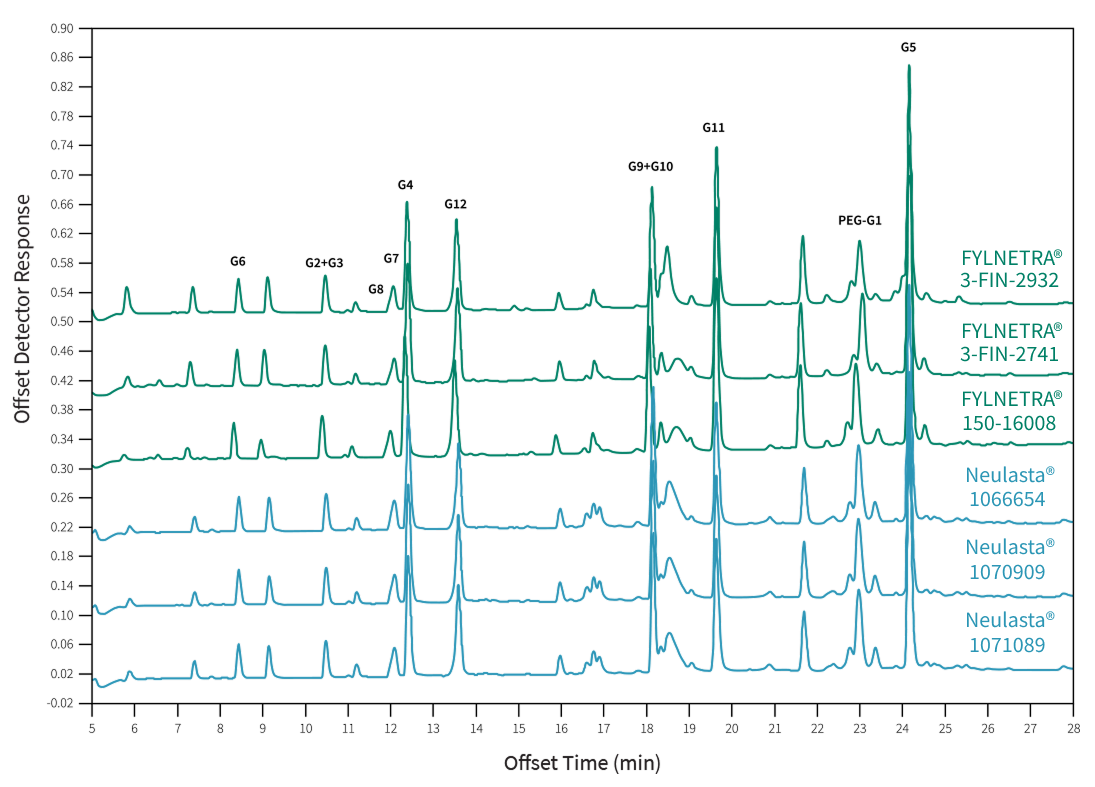
The post-translational modification analyses demonstrated a similar relative content of acidic and basic forms between both proteins.
Similar protein content and high purity was found in both FYLNETRA® and Neulasta®.
Comparative accelerated and forced degradation studies have shown a similar behavior and degradation profile of FYLNETRA® and Neulasta®.
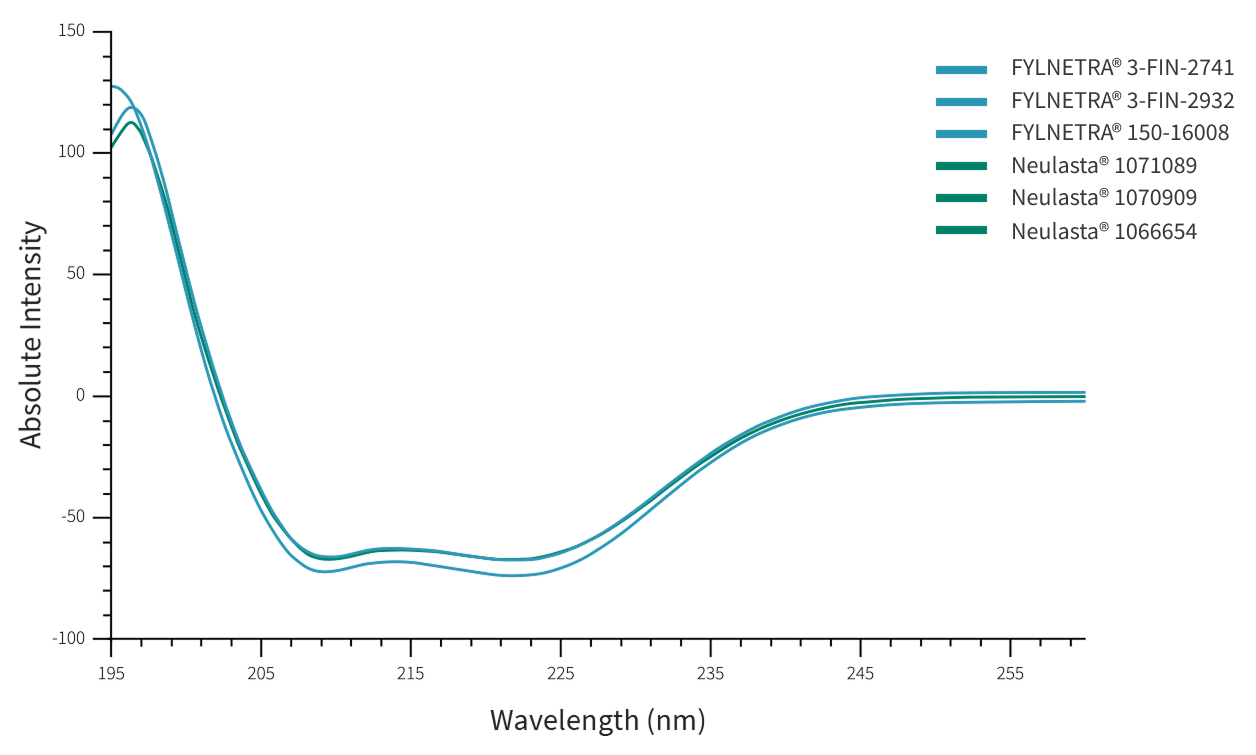
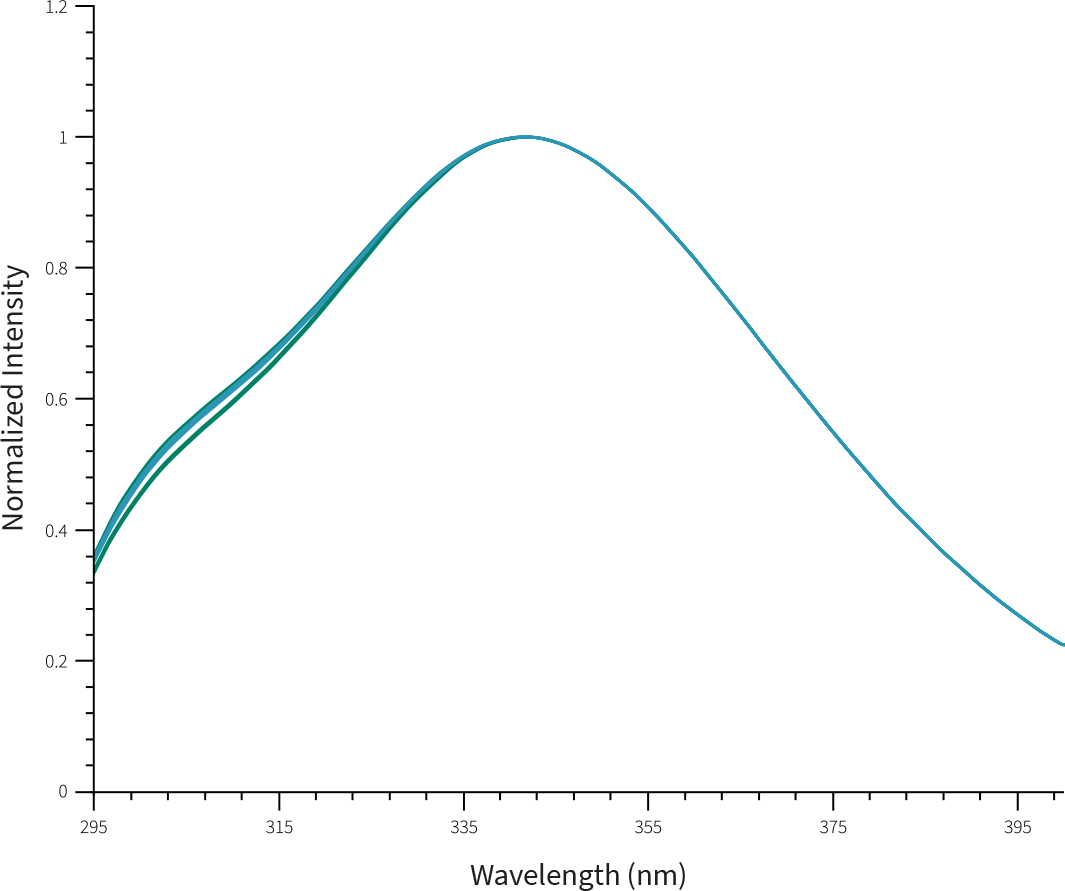
Similarity between FYLNETRA® and Neulasta® in terms of molecular conformation was demonstrated as seen by identical Far-UV Circular Dichroism Spectra, and Intrinsic Fluorescence spectra.
Representative Far-UV Circular Dichroism Spectra of FYLNETRA® and Neulasta®
Overlay of Representative Intrinsic Fluorescence Spectra of FYLNETRA® and Neulasta®
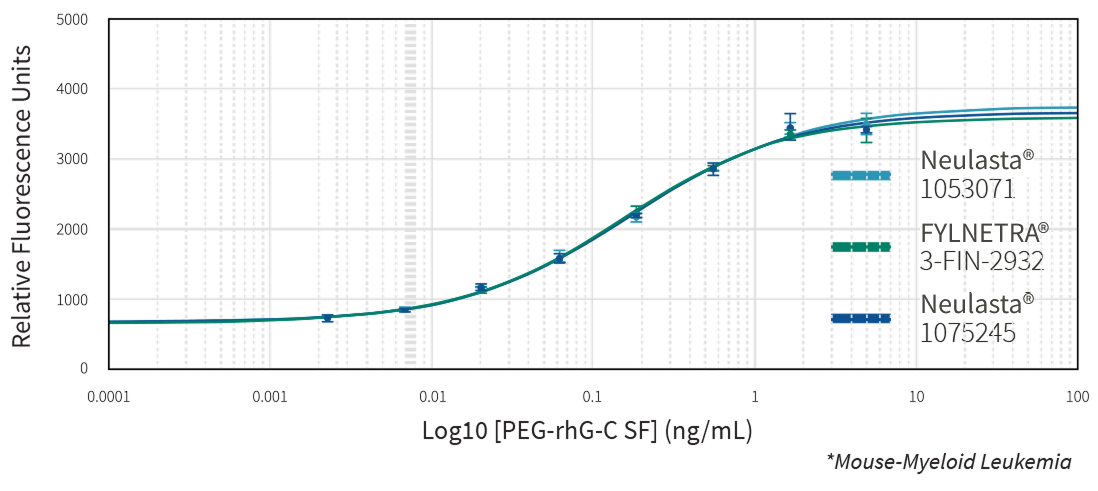
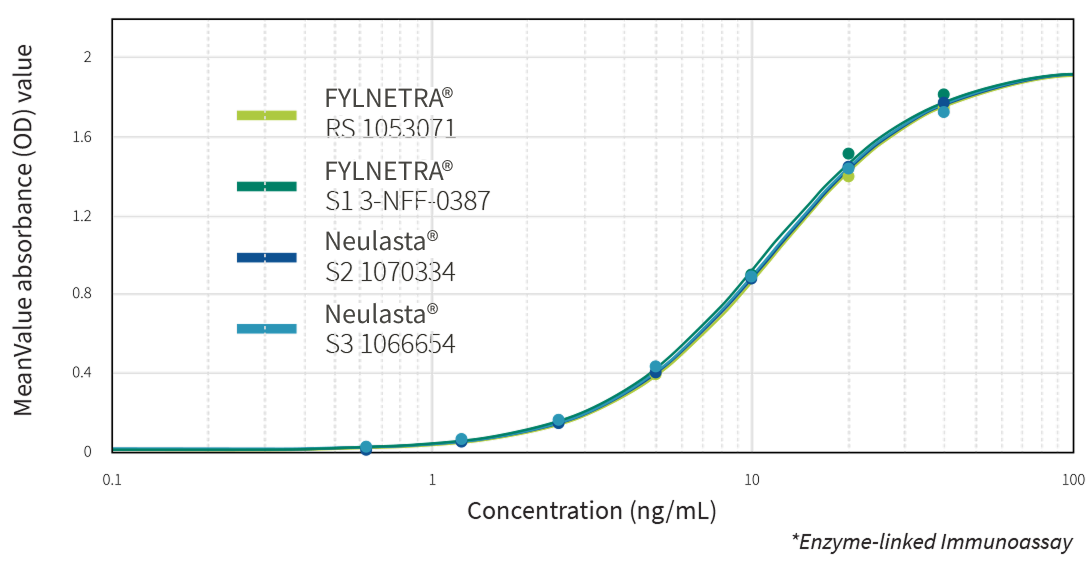
Multiple state-of-the-art orthogonal methods demonstrated the similarity of FYLNETRA® to its reference product regarding biological activity. Highly similar potency of FYLNETRA® and Neulasta® relative to filgrastim reference standard was seen in the M-NFS-60 Cell Proliferation Assay.
Representative Potency Dose Response Curve Showing Similar Potency of FYLNETRA® and Neulasta® in M-NFS-60* Cell Proliferation Assay
Comparative Results of Receptor Binding by ELISA*:
Representative ELISA Dose Response Curve for FYLNETRA® Versus Neulasta®
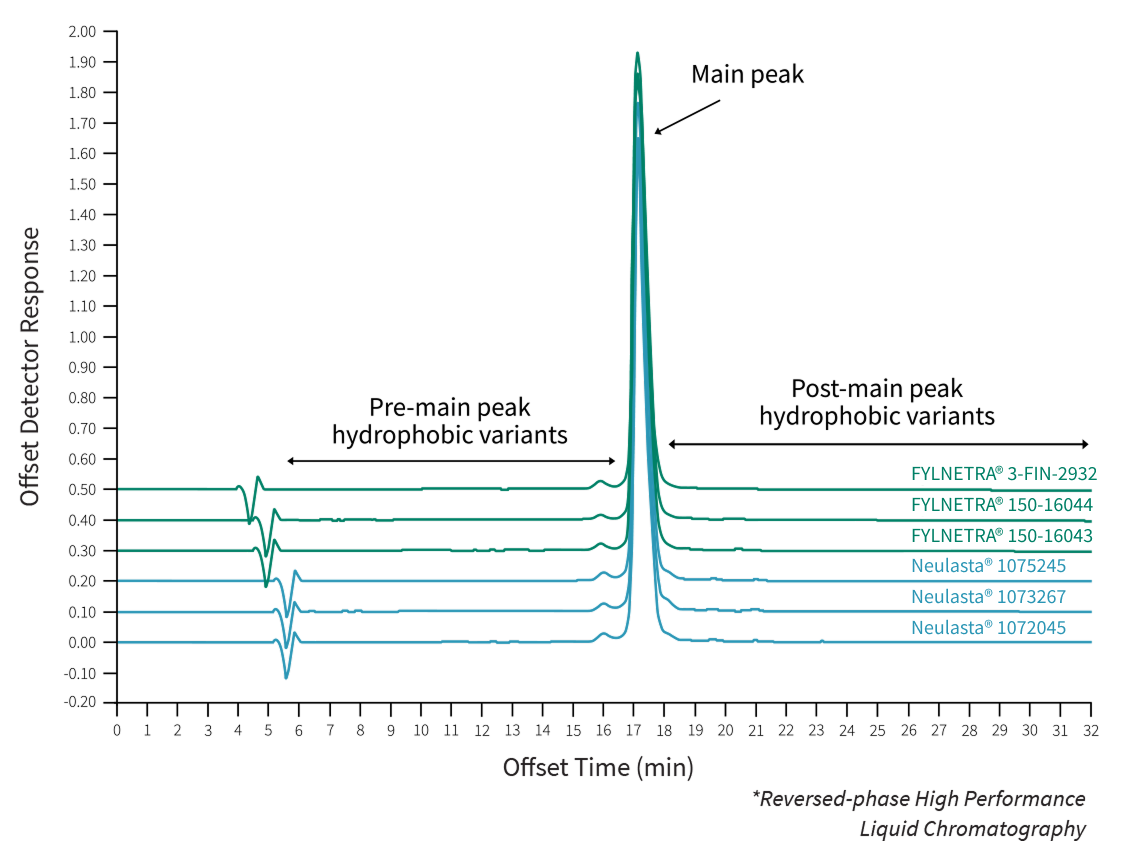
Comparative Results of RP-HPLC* Chromatography of FYLNETRA® and Neulasta®
References:
1. Fylnetra® Summary Basis of Approval – Drug Approval Package https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761084Orig1s000TOC.cfm
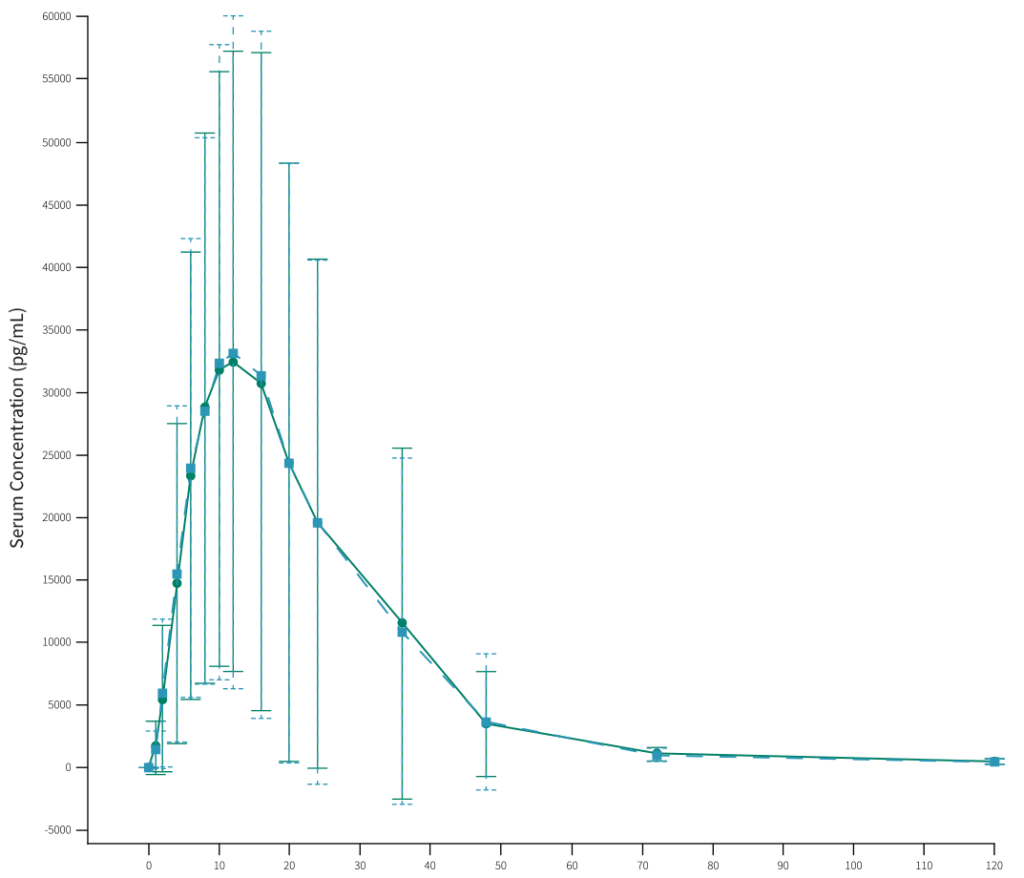
One PK/PD study was carried out in healthy volunteers’ to investigate and compare the PK profiles of FYLNETRA® and Neulasta® and confirm bioequivalence between both products:
The mean serum PEG-rhG-CSF* concentration versus time profiles following a single SC injection of 2mg FYLNETRA® (TPI-120) and Neulasta® are shown in a linear scale.

References:
1. Fylnetra® Summary Basis of Approval – Drug Approval Package https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761084Orig1s000TOC.cfm

Table 23 TPI-CL-109-A: Summary of TEAEs in ≥ 2 Subjects in the TPI-120 Treatment Arm (Safety Population)4
FMQ* |
Study TPI-CL-109-A |
|
|---|---|---|
TPI-120 |
US-Neulasta® |
|
All subjects |
60 (50.4%) |
56 (50.5%) |
Back pain |
30 (25.2%) |
31 (27.9%) |
Headache |
18 (15.1%) |
17 (15.3%) |
Myalgia |
6 (5.0%) |
2 (1.8%) |
Pain in extremity |
4 (3.4%) |
6 (5.4%) |
Arthralgia |
3 (2.5%) |
2 (1.8%) |
Local administration reaction |
2 (1.7%) |
4 (3.6%) |
Abdominal pain |
2 (1.7%) |
0 |
Upper respiratory tract |
2 (1.7%) |
0 |
Pain |
2 (1.7%) |
1 (0.9%) |
FMQ* |
All subjects |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
60 (50.4%) |
56 (50.5%) |
|
FMQ* |
Back pain |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
30 (25.2%) |
31 (27.9%) |
|
FMQ* |
Headache |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
18 (15.1%) |
17 (15.3%) |
|
FMQ* |
Myalgia |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
6 (5.0%) |
2 (1.8%) |
|
FMQ* |
Pain in extremity |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
4 (3.4%) |
6 (5.4%) |
|
FMQ* |
Arthralgia |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
3 (2.5%) |
2 (1.8%) |
|
FMQ* |
Local administration reaction |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
2 (1.7%) |
4 (3.6%) |
|
FMQ* |
Abdominal pain |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
2 (1.7%) |
0 |
|
FMQ* |
Upper respiratory tract infection |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
2 (1.7%) |
0 |
|
FMQ* |
Pain |
|
|---|---|---|
Study TPI-CL-109-A |
TPI-120 |
US-Neulasta |
2 (1.7%) |
1 (0.9%) |
|
*Grouped Terms by FDA Medical Query (FMQ).
Incidences are based on the number of subjects, not the number of events. Although a subject may have had 2 or more clinical AEs, the subject is counted only once in a category. The same subject may appear in different categories.
[Source: ADAE.xpt and ADSL.xpt]
References:
1. Fylnetra® Summary Basis of Approval – Drug Approval Package https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761084Orig1s000TOC.cfm
2. Food and Drug Administration, FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Guidance for Industry, 2015.
3. Food and Drug Administration, FDA. Draft Guidance: Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations, 2019.
4. Fylnetra® Full Prescribing Information – https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eeadd641-573d-47fe-897a-61006e5f9e03
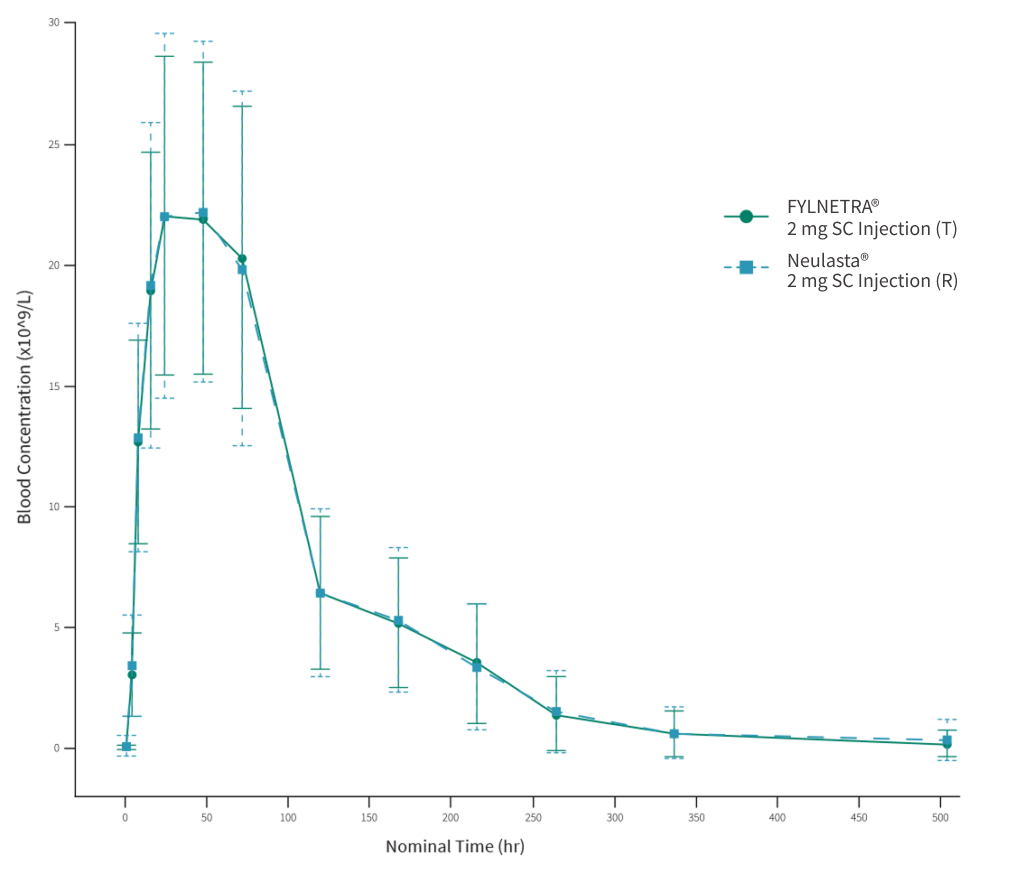
The PK/PD study provided the comparative PD characteristics that established PD similarity and hence comparative clinical efficacy for FYLNETRA® versus Neulasta®.
PD concentration time curve shown below revealed the peak and overall baseline-corrected blood ANC (as measured by geometric mean Emax and AUEC(0-t) were similar following a SC injection of either FYLNETRA® (TPI-120) or Neulasta®, as shown by the less than 10% difference in Emax and AUEC(0-t) between treatments.
The 90% CIs around the GMR of blood ANC Emax and AUEC(0-t) for TPI-120 relative to Neulasta® were within the limits of the 80.00% – 125.00% boundary, and hence PD bioequivalence was demonstrated.

References:
1. Fylnetra® Summary Basis of Approval – Drug Approval Package https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761084Orig1s000TOC.cfm
2. Food and Drug Administration, FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Guidance for Industry, 2015.
3. Food and Drug Administration, FDA. Draft Guidance: Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations, 2019.
4. Fylnetra® Full Prescribing Information – https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eeadd641-573d-47fe-897a-61006e5f9e03